In global medical research, the reliability and professionalism of biosafety equipment remain critical to ensuring the safe execution of scientific experiments. Triesta Sciences has recently equipped its clinical and molecular biology laboratory with the Haier Biomedical Biosafety Cabinet HR1200-IIA2-N, providing robust protection for its research activities.

Client Profile: Triesta Sciences and Its Role in the Industry
Triesta Sciences is a division of Healthcare Global Enterprises (HCG), a leading diagnostic center affiliated with India's network of top oncology and multi-specialty hospitals. Headquartered in Bangalore, HCG operates 22 comprehensive cancer care centers nationwide. Demands and Challenges: Reinstalling Old Equipment in a New Lab Setting Haier Biomedical's partner Biotra has been involved in the laboratory project from the very beginning. As part of the new facility's setup, Class II A2 biosafety cabinets were required. However, previously used units faced installation challenges due to the new laboratory's limited ceiling height of just 8 feet. These models could not be installed without structural modifications; unauthorized modifications could invalidate the products' existing NSF certification, making them non-compliant with safety standards. Solution: Precise Through Technical Matching and Multi-Party Collaboration To overcome the installation challenge, Biotra promptly proposed the Haier Biomedical HR1200-IIA2-N Class II Biosafety Cabinet as a solution. This NSF-certified model is equipped with ULPA filters, featuring compact dimensions and advanced airflow design. Through multiple rounds of technical discussions and solution alignment, Biotra aligned with key stakeholders, including leads in genomics, clinical laboratories, and molecular biology departments, as well as facilities and procurement teams. By prioritizing certification integrity, design suitability, and international quality benchmarks, Biotra outperformed local alternatives, establishing Haier Biomedical as the optimal solution for this mission-critical laboratory project. Project Milestones: Fulfillment Confirmed and Equipment Deployment Underway The project has already made notable progress. The end customer has confirmed the purchase of four Haier Biomedical HR1200-IIA2-N Biosafety Cabinets. One unit has already been successfully installed in the reference laboratory, while the remaining three are currently in transit and scheduled for installation soon. This collaboration fully demonstrates the strength of Haier Biomedical's product solutions, the strategic technical support and sales coordination provided by its partner Biotra, and their joint ability to deliver certified, compliant, and customized biosafety solutions even within highly constrained facility environments.



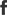





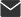



.png)